Diabetic Neuropathy
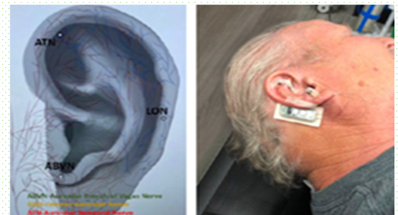
NeuroSolutions 100 is an FDA-approved Class II, minimally invasive vagus and trigeminocervical nerve stimulator designed for patients with diabetic neuropathy and associated pain.
The device is worn for 20 days and administered through a quick, in-office 20-minute procedure—providing a non-pharmacologic option for long-term pain relief. Clinical results show up to 87% pain reduction lasting six months after treatment.
Advantages:
- Minimally invasive with rapid procedure (20 minutes in office)
- Demonstrated pain reduction up to 87% for six months*
- Short device wear period (20 days)
- Pre-authorization managed directly by the company
- Covered by Medicare and most commercial plans with excellent reimbursement